Abstract
Agents that can improve the function and/or numbers of hematopoietic progenitor and stem cells (HSC) are of great importance for the treatment of bone marrow failures of different etiology. However, except for the hematopoietic growth factors, which lead to significant depletion HSC via simultaneous differentiation and some anabolic steroids, the list of capable agents that can improve the function or numbers of HSC is very short. However, such drugs would have a tremendous range of application from ex vivo expansion, to bone marrow regeneration in aplastic anemia (AA) post chemotherapy, HSC transplantation or to improve the function of normal HSC in aging.
While performing a multidrug screen for the agents that could simultaneously decrease senescence and overcome proliferative block in pre-senescent cells, we have identified two compounds violuric acid (VA) and 1-naphthoquinone-2-monoxime (N2N1). Both compounds considerably extended the replicative life span (RLS) of normal cells. We applied these drugs to stromal/mesenchymal cells obtained from healthy bone marrows, primary human normal dermal fibroblasts, progeroid primary cells derived from the patients diagnosed with Werner or Bloom syndromes and small panel of cancer cell lines (SKM-1, K562, KG-1, THP-1). Both compounds, in dose dependent manner prolonged the RLS of replicatively pre-aged cells. On an average, 10-15 additional population doublings (PD) were achieved after addition of N2N1 at 1μM. VA treatment has added 8-10 extra population doublings. If compared with untreated controls that can propagate up to 45-50 PD, the treatment with VA or N2N1 adds from 16 to 30% increase in replicative life span. To compare, the effect of rapamycin (1nM) on human fibroblasts showed the RLS increase ranged from 5 to 10%. Treatment with both VA and N2N1 results in restoration of cell cycle progression, decreased activity of SAβG, down-regulation of p16, p21 and γH2A.X and, up-regulation of lamin B1 protein. Treatment with both compounds resulted in maintenance of normal telomere length.
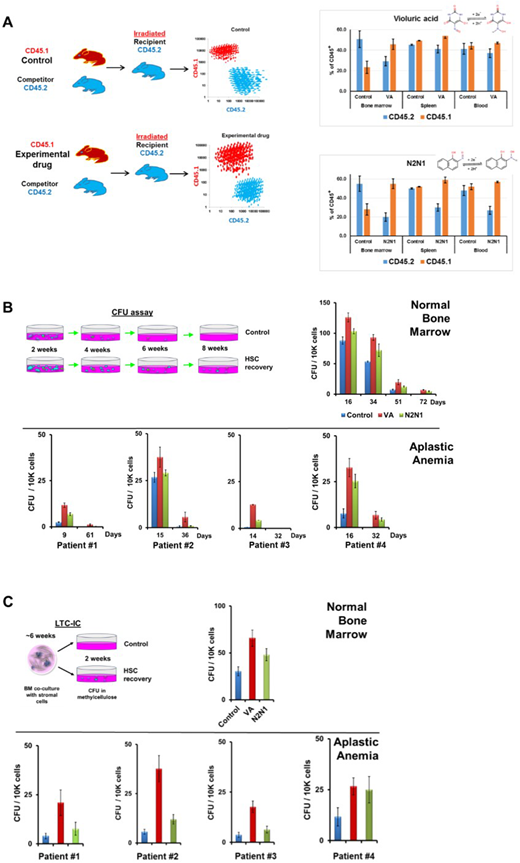
In term of HSC these agents in vivo increased the performance of HSC in competitive repopulation assay. Bone marrow cells were isolated from mice (CD45.1) treated with the vehicle or experimental drugs for three weeks. After that, they were mixed with the equal number of competitor bone marrow cells CD45.2 and injected into irradiated CD45.2 host animals. In three weeks, we observed a substantial domination of CD45.1 cells over CD45.2 in experimental groups, while control (vehicle) group exhibited equal representation of both genotypes. In vitro, treatment with VA or N2N1 contributed to prolonged availability of HSC in serial replating CFU assays in methylcellulose and long-term culture initiating cell (LTC-IC) assays. Addition of VA or N2N1 to the short-term cultures (7-14 days) of normal bone marrow cells in a medium containing a cocktail of growth factors (Il6, IL3, FLT3L, TPO, SCF) resulted in maintenance and growth of HSC or progenitors. Gated on lymphocyte sub-population, treated with N2N1 or VA samples revealed ~0.3%±0.02 or 0.2% ±0.02 of CD34+, CD45+ cells correspondingly, while control samples had 0.08% of these cells (the result of three independent experiments). Most importantly, we observed colonies formation, after application of these drugs to the bone marrow isolated from the patients diagnosed with severe AA. Further studies also indicated that these agents do not promote growth of leukemic cell.
Analysis of mechanism of action showed that VA and N1N2 function as redox co-factors in oxidations of NAD(P)H. VA transfers electrons non-enzymaticly from NAD(P)H to oxidized glutathione or peroxides. N2N1 is a redox co-factor for the NAD(P)H dehydrogenase (quinone) 1 (NQO1) and together they move electrons from NAD(P)H to cytochrome c or CoQ10. As such, we presented here a comprehensive prove that pharmacologic manipulation of redox balance controlled by glutathione or NQO1 activity via redox catalysts can ameliorate the detrimental consequences of HSC loss during normal aging by interfering with direct ROS mediated signaling or attenuating collateral ROS mediated damages.
Maciejewski:Ra Pharmaceuticals, Inc: Consultancy; Apellis Pharmaceuticals: Consultancy; Ra Pharmaceuticals, Inc: Consultancy; Alexion Pharmaceuticals, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Apellis Pharmaceuticals: Consultancy; Alexion Pharmaceuticals, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal